Antibody titers to rule them all
Goel, Painter, Apostolidis, and Mathew et al. 2021 1 mRNA Vaccination Induces Durable Immune Memory to SARS-CoV-2 with Continued Evolution to Variants of Concern
I was looking through the FDA briefing document for the upcoming advisory committee meeting on the Pfizer 3rd dose booster shots for their COMIRNATY vaccine [previously known as BNT162b2]. I found it a little lighter on data than I expected, with the primary focus on antibody levels in the blood measured 1 month after injection, known as antibody titers.
The sample size is modest, 306 in the Phase II/III subset, and while there is the required adverse event data, there is nothing at all on what was the primary endpoint for the original study, effectiveness against symptomatic disease. The working theory seems to be that the surrogate measure of antibody titer is the most important thing.
I think this illustrates something really important about the rancor around vaccines and how they have been approved and rolled out in the United States, but what I’m going to discuss may apply elsewhere as well.
I will offer my standard disclaimer, this constitutes my personal opinion, insofar as I am not representing any institution here, but it is also my professional opinion, as skilled engineer and statistician within this field. I am not qualified to diagnose disease, but I am qualified to discuss the data and its implications.
Jennifer Block published an editorial in the BMJ Vaccinating people who have had covid-19: why doesn’t natural immunity count in the US?
Block documents in that editorial that many of the key decision makers and influencers on vaccine policy within the United States, people like Anthony Fauci and Peter Marks, largely focus on antibody levels, something readily measured using widely available test technology.
In April, Anthony Fauci told US radio host Maria Hinajosa that people who have had covid-19 (including Hinajosa) still need to be “boosted” by vaccination because “your antibodies will go sky high.”
…
In June, Peter Marks, director of the Food and Drug Administration’s Center for Biologics Evaluation and Research, which regulates vaccines, went a step further and stated: “We do know that the immunity after vaccination is better than the immunity after natural infection.” In an email, an FDA spokesperson said Marks’s comment was based on a laboratory study of the binding breadth of Moderna vaccine induced antibodies. The research did not measure any clinical outcomes. Marks added, referring to antibodies, that “generally the immunity after natural infection tends to wane after about 90 days.”
…
The John Snow memorandum, written in response (with signatories including Rochelle Walensky, who went on to head the CDC), stated “there is no evidence for lasting protective immunity to SARS-CoV-2 following natural infection.” That statement has a footnote to a study of people who had recovered from covid-19, showing that blood antibody levels wane over time.
Here are the references cited by Marks Antibodies elicited by mRNA-1273 vaccination bind more broadly to the receptor binding domain than do those from SARS-CoV-2 infection and the John Snow memorandum A comprehensive, longitudinal analysis of humoral responses specific to four recombinant antigens of SARS-CoV-2 in severe and non-severe COVID-19 patients. Both studies were used to defend a statement that natural immunity wanes faster than immunity elicited by a vaccine. Neither looked at anything but antibody titers. Other elements of the immune system were not studied in those experiments.
However, there is considerable debate among experts as to what does offer a complete picture of our immune system’s response to disease. Antibodies are of course an important part of the immune system, but there are also other major components, such as T-cells and B-cells. I suspect one part of this is simple convenience: antibody levels are readily measured with a blood sample, and the decay of antibody levels over time follows a predictable pattern.
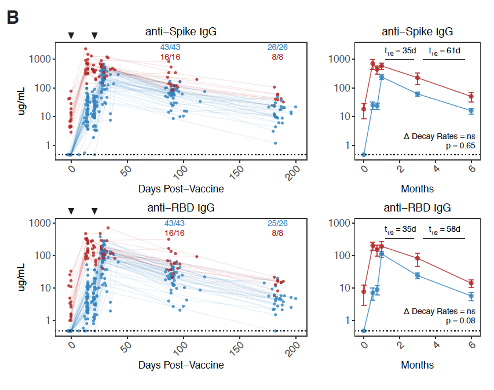
Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection
T-cell and B-cell activation can also be measured, but it is harder to do and slower. But not a lot harder. The technology is also readily available. Studies have been done, both on surrogates like the activity of T-cells and B-cells, and more directly against what would count as a clinical endpoint. For example Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection or Quantifying the risk of SARS-CoV-2 reinfection over time.
Recently, two senior reviewers in the Office of Vaccines at the FDA, Philip Krause and Marion Gruber, resigned their positions. Back channel reports cited political interference in the vaccine approval process as the reason for their resignations. Now, Krause and Gruber have explained their position in the Lancet Considerations in boosting COVID-19 vaccine immune responses.
One of the key disagreements cited in the Lancet letter is that evidence based upon antibody titer alone does not necessarily predict protection against infection or hospitalization. Krause and Gruber, along with their co-authors, cite evidence about T-cell and B-cell activity, as well as the observational studies that have been done on vaccine effectiveness in a variety of formats.
Looking at all this together, my interpretation is that in the United States at least, policy decisions appear to heavily weight antibody titer as the most important piece of data. The statements noted above, in combination with the booster vaccine briefing document certainly support this. Then if you add in the fact that two very senior FDA reviewers who wrote an article from a dissenting point of view resigned their posts at about the time booster shots are coming up for review.
In one sense, this is an intra-science debate, with one group focusing on a key metric, and others arguing for a more holistic appraisal. But this very much matters for the public as well. Right now, the official position in the United States is vaccine-mediated immunity is considered superior to natural immunity for the reasons cited above. A consequence of this is an insistence that anyone who has had COVID would still be required to be vaccinated under recently announced vaccine mandates.
However, if the opposing position is true, that natural immunity also offers long-term resistance to disease caused by SARS-CoV-2, then for the portion of the population who have some degree of natural immunity, the risk-benefit analysis is harder to justify. At least in theory, medical treatments in the United States are expected to demonstrate the benefits exceed the risk. In this case, if there is some degree of natural immunity, the benefits of vaccination would be reduced or even eliminated, leaving only the risks inherent to the therapy.


Comments ()